CRANIAL NERVES
Definition: Cranial nerves are nerves that emerge directly from the brain stem in contrast to spinal nerves which emerge from segments of the spinal cord.
Some of these nerves bring information from the sense organs to the brain; some control muscles; and other are connected to glands or internal
organs such as the heart, lungs, colon, etc.
There are neurologists in the world specialised on Rett syndrome who believe that the
Cranial nerves are badly functioning in this disorder and that they are the cause of the most important symptoms. Thinking about: strabism,
breathing and swallowing difficulties, dribling, teeth grinding, reflux and constipation. I am sharing their opinion from my own experience.
Therefore I want to present here an anatomic schema that shows the role of the Cranial nerves in the body. Is it accidental that this nerves
are attached to most of organs that have trouble functioning in the Rett syndrome? I will leave it to the reader himself to draw a conclusion
on this issue from their own experience with the girls they know.
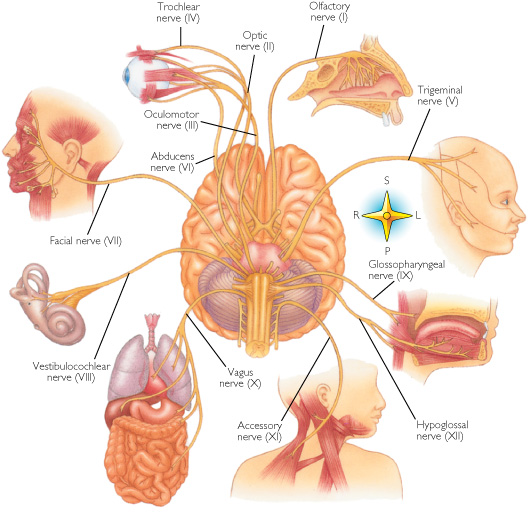
Cranial nerve I: Olfactory nerve
This is a pure sensory nerve fiber.
Cranial nerve II: Optic nerve
It carries visual information to the brain. This is a pure sensory nerve fiber.
Cranial nerve III: Oculomotor nerve
This is a pure motor nerve. It provides somatic motor innervation to four of the extrinsic eye muscles: the superior rectus, inferior rectus, medial rectus, and the inferior oblique muscles. It also innervates the muscles of the upper eyelid and the intrinsic eye muscles Together, CN III, CN IV and CN VI control the six muscles of the eye.
Cranial nerve IV: Trochlear nerve
Provides pure motor innervation to the superior oblique eye muscle.
Cranial nerve V: Trigeminal nerve
This is the largest cranial nerve. It provides sensory information from the face, forehead, nasal cavity, tongue, gums and teeth (touch, and temperature) and provides somatic motor innervation to the muscles of mastication or “chewing”.
Cranial nerve VI: Abducens nerve
The abducens nerve carries pure motor innervation to lateral rectus eye muscle.
Cranial nerve VII: Facial nerve
It carries somatic motor innervation to the many muscles for facial expression. It carries sensory information form the face (deep pressure sensation) and taste information. It is composed of both sensory and motor axons.
Cranial nerve VIII: Vestibulocochlear nerve
It carries vestibular information to the brain from the inner ear providing the sense of balance and the sense of hearing. It is pure sensory nerve fiber.
Cranial nerve IX: Glossopharyngeal nerve
It carries sensory information (touch, temperature, and pressure) from the pharynx and soft palate. It carries taste sensation from the taste buds on the posterior one third of the tongue. It provides somatic motor innervation to the throat muscles involved in swallowing. It provides visceral motor innervation to the salivary glands. This cranial nerve also supplies the carotid sinus and reflex control to the heart . It is composed of both sensory and motor axons.
Cranial nerve X: Vagus nerve
It is the longest cranial nerve innervating many structures in the throat, including the muscles of the vocal cords, thorax and abdominal cavity. It provides sensory information (touch, temperature and pressure) from the external auditory meatus (ear canal) and a portion of the external ear. It carries taste sensation and sensory information from the esophagus, respiratory tract, and abdominal viscera (stomach, intestines, liver, etc.). It provides visceral motor innervation to the heart, stomach, intestines, and gallbladder. It is composed of both sensory and motor axons.
Cranial nerve XI: Spinal Accessory nerve
It has two branches. The cranial branch provides somatic motor innervation to some of the muscles in the throat involved in swallowing. The spinal branch provides somatic motor innervation to the trapezius muscles, providing muscle movement for the upper shoulders head and neck. It is pure motor nerve fiber.
Cranial nerve XII: Hypoglossal nerve
It provides somatic motor innervation to the muscles of the tongue. This pure motor nerve.
Problems with nerves 3, 4 and 6
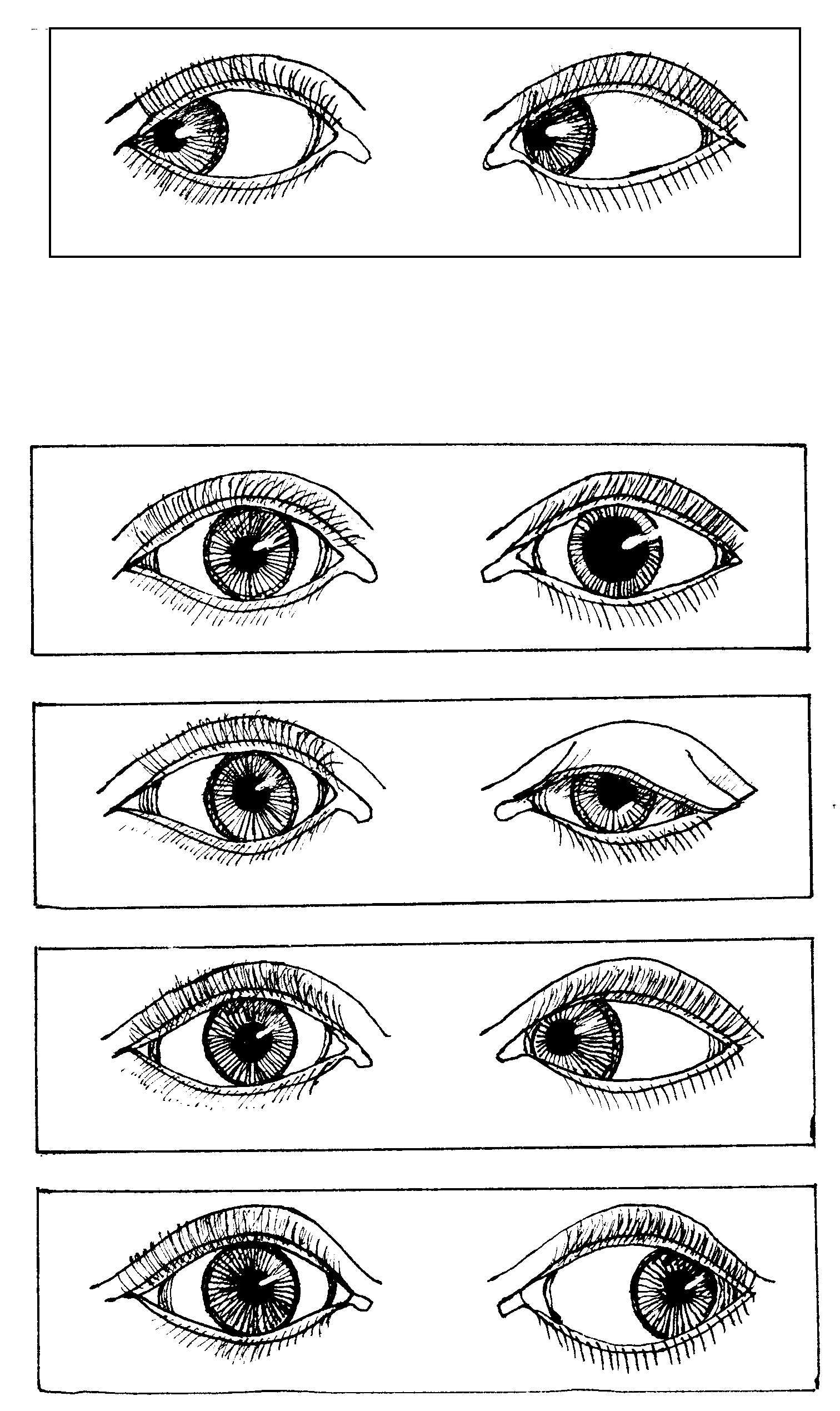 ________
________
 Problems with nerve 12
Problems with nerve 12
Problems with nerve 11
Facial Cranial nerve VII ___ 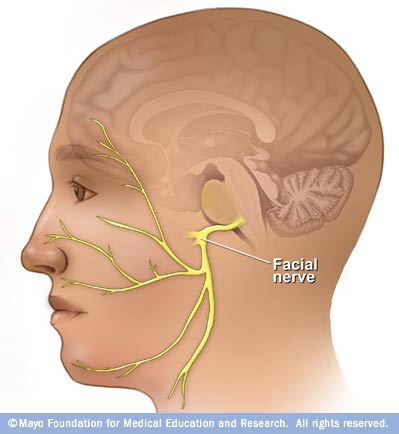
RELATED RESEARCH
Breathing dysfunction in Rett syndrome:
understanding epigenetic regulation of the respiratory network
Ogier M, Katz DM. Department of Neurosciences, Case Western Reserve University School of Medicine,
10900 Euclid Avenue, Cleveland, OH 44106-4975, USA.
Severely arrhythmic breathing is a hallmark of Rett syndrome (RTT) and profoundly
affects quality of life for patients and their families. The last decade has seen the
identification of the disease-causing gene, methyl-CpG-binding protein 2 (Mecp2)
and the development of mouse models that phenocopy many aspects of the human syndrome,
including breathing dysfunction. Recent studies have begun to characterize the breathing
phenotype of Mecp2 mutant mice and to define underlying electrophysiological and neurochemical deficits.
The picture that is emerging is one of defects in synaptic transmission throughout
the brainstem respiratory network associated with abnormal expression in several neurochemical
signaling systems, including brain-derived neurotrophic factor (BDNF),
biogenic amines and gamma-amino-butyric acid (GABA). Based on such findings, potential
therapeutic strategies aimed at improving breathing by targeting deficits in neurochemical signaling are being explored.
This review details our current understanding of respiratory dysfunction and underlying mechanisms
in RTT with a particular focus on insights gained from mouse models.
Respir Physiol Neurobiol. 2008 Dec 10
Breathing dysfunctions associated with impaired control
of postinspiratory activity in Mecp2-/y knockout mice.
Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M.
Department of Pediatrics and Pediatric Neurology, Georg August University, Robert-Koch-Str. 40, 37075 Göttingen, Germany
Rett syndrome (RTT) is an inborn neurodevelopmental disorder caused by mutations in the X-linked
methyl-CpG binding protein 2 gene (MECP2). Besides mental retardation, most patients suffer from
potentially life-threatening breathing arrhythmia. To study its pathophysiology, we performed comparative
analyses of the breathing phenotype of Mecp2-/y knockout (KO) and C57BL/6J wild-type mice using the perfused
working heart-brainstem preparation (WHBP). We simultaneously recorded phrenic and efferent vagal nerve
activities to analyse the motor pattern of respiration, discriminating between inspiration,
postinspiration and late expiration. Our results revealed respiratory disturbances in KO preparations
that were similar to those reported from in vivo measurements in KO mice and also to those seen in RTT patients.
The main finding was a highly variable postinspiratory activity in KO mice that correlated closely with
breathing arrhythmias leading to repetitive apnoeas even under undisturbed control conditions.
Analysis of the pontine and peripheral sensory regulation of postinspiratory activity in KO preparations revealed:
(i) prolonged apnoeas associated with enhanced postinspiratory activity after glutamate-induced activation of
the pontine Kölliker-Fuse nucleus; and (ii) prolonged apnoeas and lack of reflex desensitization in response
to repetitive vagal stimulations. We conclude that impaired network and sensory mediated synaptic control of
postinspiration induces severe breathing dysfunctions in Mecp2-/y KO preparations. As postinspiration
is particularly important for the control of laryngeal adductors, the finding might explain the upper
airway-related clinical problems of patients with RTT such as apnoeas, loss of speech and
weak coordination of breathing and swallowing.
J Physiol. 2007 Mar 15;J Physiol. 2007 Oct 1
PMID: 17204503 [PubMed - indexed for MEDLINE]
Functional evidence of brain stem immaturity in Rett syndrome.
Julu PO, Kerr AM, Hansen S, Apartopoulos F, Jamal GA.
Peripheral Nerve and Autonomic Unit INS, Southern General Hospital, Glasgow, Scotland.
Autonomic activity and respiration were studied in Rett syndrome (RS) and age matched controls.
Breathing movements were monitored using a pletysmograph around the chest. Sympathetic activity was
monitored by measuring blood pressure (BP) using the Finapres. Cardiac parasympathetic activity was
monitored by measuring the cardiac response to baroreflex using the NeuroScope which outputs measure
of cardiac vagal tone (CVT) in units of a linear vagal scale (LVS).
Resting CVT (means +/- SEM) was 10.5 +/- 0.9 units in the LVS and BP was 94.6 +/- 6.4 mmHg in controls.
The BP was 78 +/- 4.33 mmHg and CVT was 3.6 +/- 0.7 units in the LVS in girls with RS, 65% lower than
in their age matched controls (p < 0.001), but equal to previously reported level in neonates.
Each girl with RS had at least 6 types of breathing dysrhythmias, a sign of instability of the respiratory oscillator.
The sympathetic system controlled the HR and BP smoothly during breath holding in control girls,
but there were oscillations and rebounds in RS. The HR and BP were under parasympathetic influence
during hyperventilation in normal girls but not in RS. The CVT was invariably withdrawn at the height
of sympathetic activity during both hyperventilation and breath holding in RS, leading to sympathovagal
imbalance with the risk of cardiac arrhythmias and possibly sudden death. Neonatal level of CVT,
poor autonomic integration and multiple breathing dysrhythmias shows medullary immaturity in RS.
It is the first demonstration of immaturity of the brain which could be used for screening in early
childhood and potentially useful for diagnosis and management of RS.
Eur Child Adolesc Psychiatry. 1997;
PMID: 9452920 [PubMed - indexed for MEDLINE]
Functional evidence of brain stem immaturity in Rett syndrome.
Julu PO, Kerr AM, Hansen S, Apartopoulos F, Jamal GA.
Peripheral Nerve and Autonomic Unit INS, Southern General Hospital, Glasgow, Scotland.
Autonomic activity and respiration were studied in Rett syndrome (RS) and age matched controls.
Breathing movements were monitored using a pletysmograph around the chest. Sympathetic activity was
monitored by measuring blood pressure (BP) using the Finapres. Cardiac parasympathetic activity was
monitored by measuring the cardiac response to baroreflex using the NeuroScope which outputs measure
of cardiac vagal tone (CVT) in units of a linear vagal scale (LVS).
Resting CVT (means +/- SEM) was 10.5 +/- 0.9 units in the LVS and BP was 94.6 +/- 6.4 mmHg in controls.
The BP was 78 +/- 4.33 mmHg and CVT was 3.6 +/- 0.7 units in the LVS in girls with RS, 65% lower than
in their age matched controls (p < 0.001), but equal to previously reported level in neonates.
Each girl with RS had at least 6 types of breathing dysrhythmias, a sign of instability of the respiratory oscillator.
The sympathetic system controlled the HR and BP smoothly during breath holding in control girls,
but there were oscillations and rebounds in RS. The HR and BP were under parasympathetic influence
during hyperventilation in normal girls but not in RS. The CVT was invariably withdrawn at the height
of sympathetic activity during both hyperventilation and breath holding in RS, leading to sympathovagal
imbalance with the risk of cardiac arrhythmias and possibly sudden death. Neonatal level of CVT,
poor autonomic integration and multiple breathing dysrhythmias shows medullary immaturity in RS.
It is the first demonstration of immaturity of the brain which could be used for screening in early
childhood and potentially useful for diagnosis and management of RS.
Eur Child Adolesc Psychiatry. 1997;
PMID: 9452920 [PubMed - indexed for MEDLINE]
Sympathetic overactivity and plasma leptin levels in Rett syndrome
Acampa M, Guideri F, Hayek J, Blardi P, De Lalla A, Zappella M, Auteri A.
Department of Clinical Medicine and Immunological Sciences, Section of Internal Medicine,
University of Siena, Siena, Italy. M.Acampa@ao-siena.toscana.it
Rett syndrome (RTT) is a severe developmental-neurological disorder, characterized by profound
and progressive loss of intellectual functioning, occurring after a period (of at least 6 months)
of normal development with classic stereotype hand movements, gait ataxia, jerky truncal ataxia,
deceleration of brain and body organ growth and cardiac dysautonomia. Pathogenesis of sympathetic
overactivity in RTT is unknown, but a previous study observed increased plasma leptin levels in
Rett girls and it is well known the role of leptin in the regulation of sympathetic nervous system activity.
Aim of our study is to evaluate a relationship between plasma leptin levels and sympathetic activity in RTT.
Thirty-two female patients (12.1+/-6.3 years), affected by RTT were enrolled in the study. In all the subjects,
we analyzed heart rate variability, QT corrected interval and plasma leptin levels. A significant correlation
was found between plasma leptin levels and LF/HF (expression of sympatho-vagal balance) (Spearman r=0.44, p=0.001).
There is also a significant negative correlation between HF component (expression of vagal activity)
and plasma leptin levels (Spearman r=-0.037, p=0.03) and a positive correlation between LF component and
plasma leptin levels (Spearman r=0.047, p=0.01). These results show that in RTT higher plasma leptin levels
appear to be associated with sympathetic overactivity, suggesting a role for leptin in cardiac dysautonomia.
Neurosci Lett. 2008 Feb 13;
PMID: 18226448 [PubMed - indexed for MEDLINE]
|
|


 ________
________
 Problems with nerve 12
Problems with nerve 12
